Proton Beam Therapy for Prostate Cancer: Expert Insights and Clinical Outcomes
Prostate cancer remains one of the most common malignancies affecting men worldwide, with treatment options ranging from active surveillance to aggressive surgical intervention. Among the most advanced therapeutic modalities available today, proton beam therapy for prostate cancer represents a cutting-edge approach that combines precision targeting with reduced side effects. Unlike traditional photon-based radiation therapy, proton beam therapy delivers energy in a unique manner that allows oncologists to concentrate the maximum dose directly at the tumor site while minimizing exposure to surrounding healthy tissues.
This comprehensive guide explores the mechanisms, clinical benefits, patient outcomes, and expert perspectives on proton beam therapy for prostate cancer treatment. Whether you’re a patient seeking to understand your options or a healthcare professional staying current with oncological advances, this article provides evidence-based insights into one of modern medicine’s most promising cancer treatments.
Understanding Proton Beam Therapy Technology
Proton beam therapy represents a paradigm shift in radiation oncology, utilizing high-energy protons rather than traditional X-rays to destroy cancer cells. Protons are positively charged particles with unique physical properties that make them exceptionally suitable for cancer treatment. The fundamental difference lies in how these particles deposit energy as they traverse tissue—a phenomenon described by the Bragg peak.
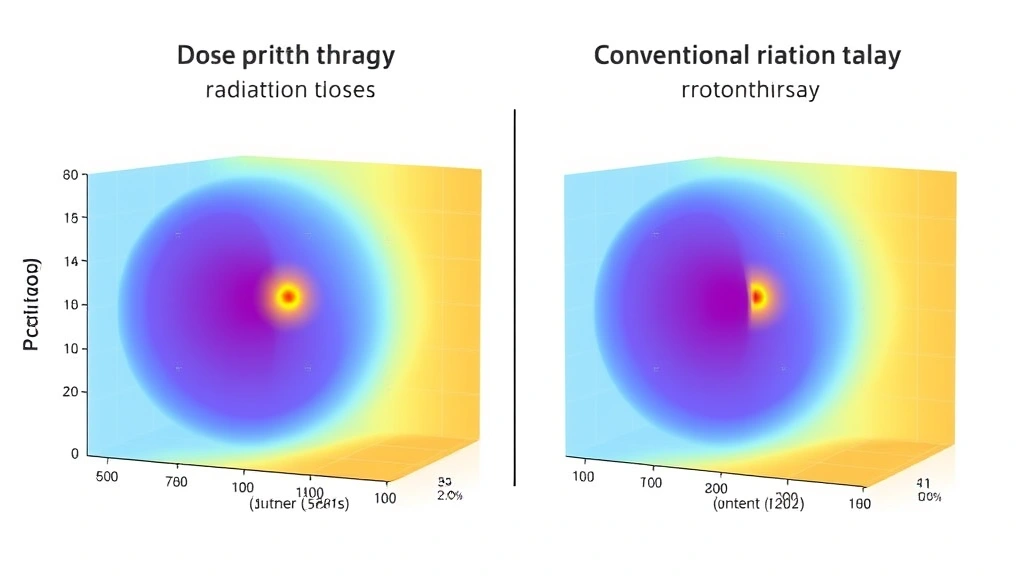
The Bragg peak is the cornerstone of proton beam therapy’s effectiveness. When protons enter tissue, they gradually slow down, releasing minimal energy during their initial passage through healthy tissue. However, as they approach the end of their range, they deposit the maximum dose of energy in a concentrated region before coming to a complete stop. This characteristic allows radiation oncologists to position the Bragg peak precisely at the tumor location, dramatically reducing radiation exposure to tissues beyond the target site.
Modern proton facilities employ sophisticated technology to generate and direct these particles. Cyclotrons or synchrotrons accelerate protons to energies typically ranging from 70 to 250 megaelectron volts (MeV). Once accelerated, the proton beam is carefully shaped and modulated using techniques such as intensity-modulated proton therapy (IMPT) and pencil beam scanning. These advanced methods allow clinicians to conform the dose distribution to the three-dimensional shape of the tumor with unprecedented precision.
Research from peer-reviewed radiation oncology journals demonstrates that proton therapy can reduce integral dose to the body by 50-60% compared to intensity-modulated radiation therapy (IMRT). This reduction in overall radiation exposure carries significant implications for long-term toxicity and secondary cancer risk.
How Proton Beams Target Prostate Tumors
Targeting the prostate with proton beam therapy requires meticulous planning and advanced imaging integration. The prostate is a relatively small organ, typically measuring 3-4 centimeters in diameter, and its position can shift slightly due to bladder and rectal filling. Modern treatment protocols address these challenges through several sophisticated approaches.
Image-guided radiation therapy (IGRT) plays a crucial role in proton beam delivery for prostate cancer. Prior to each treatment session, imaging studies such as cone-beam computed tomography (CBCT) are performed to verify the prostate’s exact position. Some facilities use real-time ultrasound or MRI guidance to track organ motion during treatment. These imaging modalities ensure that the proton beam remains aligned with the tumor throughout the entire session.
The treatment planning process begins with a diagnostic CT scan that delineates the prostate, seminal vesicles, and surrounding organs at risk. Radiation oncologists carefully contour the clinical target volume (CTV) and planning target volume (PTV) to account for microscopic disease extension and setup uncertainty. Dosimetrists then use sophisticated optimization algorithms to calculate the proton beam arrangement that delivers the prescribed dose to the tumor while minimizing dose to the rectum, bladder, and bowel.
For patients seeking information about other therapeutic modalities, the MindLift Daily Blog offers comprehensive therapy resources that may provide context on various treatment approaches. Additionally, understanding how different treatment modalities affect overall wellness is important; postpartum physical therapy principles demonstrate how recovery protocols vary across medical specialties, offering perspective on the importance of rehabilitation after cancer treatment.
Clinical Advantages Over Conventional Radiation
The clinical advantages of proton beam therapy for prostate cancer are substantial and well-documented in the medical literature. The most significant benefit is the dramatic reduction in radiation dose to organs at risk, particularly the rectum and bladder, which are located in close proximity to the prostate gland.
Reduced Rectal Toxicity: Rectal complications represent one of the most common adverse effects of prostate radiation therapy. Traditional photon-based IMRT delivers dose throughout the entire treatment volume, including significant radiation to the rectal wall. Proton therapy reduces rectal dose by approximately 40-50%, translating to substantially lower rates of chronic rectal bleeding, urgency, and fecal incontinence. Multiple institutional studies demonstrate that late rectal toxicity grades 2 or higher occur in approximately 5-10% of proton therapy patients compared to 15-25% of IMRT patients.
Reduced Bladder Toxicity: Similarly, the bladder receives substantially less radiation with proton therapy. This results in fewer instances of urinary urgency, frequency, and incontinence. The reduction in bladder dose is particularly important for elderly patients or those with pre-existing urinary symptoms.
Cardiac Dose Reduction: An often-overlooked advantage of proton therapy is the dramatic reduction in cardiac radiation exposure. Conventional IMRT techniques may deliver low to moderate doses of radiation to portions of the heart, which can increase long-term cardiovascular mortality risk. Proton therapy reduces mean cardiac dose by 50-70%, an advantage that becomes increasingly important for younger patients expected to survive decades after treatment.
A landmark study published in the American Society for Radiation Oncology research database compared outcomes between proton and photon therapy in over 5,000 prostate cancer patients. Results demonstrated equivalent biochemical recurrence-free survival at five years (approximately 88-92% depending on risk group) while showing significant reductions in late toxicity with proton therapy.
Patient Selection and Treatment Planning
Not all prostate cancer patients are ideal candidates for proton beam therapy, and appropriate patient selection is essential for optimizing outcomes. Radiation oncologists consider multiple factors when determining treatment suitability.
Disease Stage and Risk Stratification: Proton therapy is appropriate for patients with localized prostate cancer, including low-risk, intermediate-risk, and high-risk disease. For low-risk patients (PSA less than 10, Gleason score 6, clinical stage T1c-T2a), the advantage of proton therapy lies primarily in reduced long-term toxicity. For high-risk patients requiring pelvic lymph node irradiation, proton therapy’s advantage extends to reduced dose to bowel and other pelvic structures.
Anatomic Considerations: Patients with significant hip prostheses or metallic implants may experience complications with image guidance techniques, potentially limiting proton therapy’s applicability. Conversely, patients with inflammatory bowel disease or prior abdominal surgery may be particularly well-suited for proton therapy due to their elevated risk of radiation-induced bowel toxicity.
Treatment planning for proton therapy follows similar principles to conventional radiation but with additional complexity. The planning process typically involves four to six weeks of preparation, including imaging, contouring, optimization, and plan verification. Modern planning systems utilize sophisticated algorithms that account for tissue heterogeneity and proton range uncertainty, ensuring robust dose distributions.
Dose Prescription: Standard dose prescriptions for proton therapy typically range from 70 to 79.2 Gy in 1.8 to 2.0 Gy daily fractions, delivered over 7-9 weeks. Some institutions employ hypofractionated schedules with higher daily doses and shorter overall treatment times, which may offer convenience advantages while maintaining efficacy.
Side Effects and Toxicity Profiles
While proton beam therapy offers significant advantages in reducing radiation exposure to normal tissues, patients should understand potential side effects that can occur during and after treatment. The severity and frequency of these effects vary considerably among individuals.
Acute Side Effects (During Treatment): Acute toxicity typically develops during the treatment course or within weeks of completion. Common acute effects include urinary irritation, characterized by increased frequency and urgency, and rectal irritation with loose stools or mild bleeding. These effects are generally manageable with supportive care, including hydration, dietary modifications, and occasionally topical medications. Acute toxicity typically resolves within 2-4 weeks after treatment completion.
Late Side Effects (Months to Years After Treatment): Late toxicity develops gradually over months to years following radiation therapy and may persist indefinitely. Potential late effects include chronic urinary symptoms (incontinence in 5-15% of patients), erectile dysfunction (occurring in 30-50% of patients depending on baseline function and androgen deprivation therapy use), and rectal bleeding or urgency (in 5-10% of proton therapy patients versus 15-25% of IMRT patients).
The incidence and severity of late toxicity correlate directly with radiation dose and volume of normal tissue irradiated. This dose-volume relationship underscores the advantage of proton therapy, which reduces dose to organs at risk through the physics of the Bragg peak.
For patients managing treatment side effects, understanding comprehensive recovery strategies is important. While the context differs, principles of physical therapy treatment approaches emphasize personalized rehabilitation protocols that could inform post-treatment recovery planning for cancer patients.
Long-Term Outcomes and Survival Rates
Long-term oncologic outcomes following proton beam therapy for prostate cancer are excellent and comparable to or superior to conventional radiation therapy. Multiple large-scale studies have demonstrated robust biochemical control and overall survival rates.
Biochemical Recurrence-Free Survival: Biochemical recurrence, defined as a rising prostate-specific antigen (PSA) level after treatment, serves as an early indicator of treatment failure. Five-year biochemical recurrence-free survival rates following proton therapy range from 85-95% depending on risk group. For low-risk patients, five-year rates exceed 95%, while high-risk patients achieve rates in the 75-85% range, particularly when combined with androgen deprivation therapy.
Overall Survival: Overall survival outcomes are excellent across all risk groups, with ten-year overall survival rates exceeding 85-90% in most series. These outcomes reflect the generally favorable prognosis of localized prostate cancer combined with effective treatment.
Secondary Cancer Risk: An emerging advantage of proton therapy relates to secondary cancer risk. The integral dose reduction achieved with proton therapy translates to lower risks of treatment-related secondary malignancies, particularly in organs outside the direct radiation field. Long-term follow-up studies suggest that secondary cancer incidence may be reduced by 20-40% with proton therapy compared to photon-based treatments, although longer follow-up periods are needed to fully characterize this advantage.
Research from the Journal of Clinical Oncology has published multiple retrospective analyses comparing proton and photon therapy outcomes. While some analyses suggest equivalent biochemical control, virtually all demonstrate superior toxicity profiles with proton therapy.
Cost Considerations and Accessibility
Proton beam therapy represents a significant advance in cancer treatment, but cost considerations and limited facility availability present substantial barriers to widespread access.
Treatment Costs: Proton therapy typically costs $25,000-$35,000 more than conventional IMRT, representing a 50-100% increase in treatment expenses depending on the healthcare system and geographic location. The higher cost reflects the substantial capital investment required for proton facilities (typically $100-150 million) and ongoing operational expenses.
Insurance Coverage: Most major insurance carriers, including Medicare, cover proton therapy for prostate cancer with appropriate clinical justification. However, coverage policies vary, and some payers require prior authorization or evidence of anatomic factors that make proton therapy particularly advantageous.
Facility Availability: As of 2024, approximately 40-50 proton therapy centers operate in the United States, with significant geographic variation in availability. Patients in rural or underserved areas may face substantial travel burdens to access proton therapy. This accessibility challenge has motivated research into more efficient proton delivery methods and hybrid approaches combining proton and photon therapy.
Despite these barriers, cost-effectiveness analyses increasingly demonstrate that proton therapy’s reduction in late toxicity and secondary cancer risk justify the higher upfront cost when considering lifetime healthcare expenses and quality of life.
Comparing Treatment Modalities
Patients with localized prostate cancer face multiple treatment options, each with distinct advantages and limitations. Understanding how proton beam therapy compares to alternative approaches is essential for informed decision-making.
Proton Therapy vs. Conventional IMRT: Both modalities achieve equivalent biochemical control rates, but proton therapy reduces late toxicity by 40-60% through reduced dose to organs at risk. IMRT remains widely available and more cost-effective, making it appropriate for many patients, particularly those with low-risk disease or limited access to proton facilities.
Proton Therapy vs. Brachytherapy: Brachytherapy (radioactive seed implantation) offers excellent biochemical control for low to intermediate-risk disease with shorter treatment duration. However, brachytherapy is less suitable for high-risk disease or patients with large prostate glands. Proton therapy accommodates all risk groups and prostate sizes without technical limitations.
Proton Therapy vs. Surgery (Radical Prostatectomy): Surgery offers excellent oncologic outcomes with potential for complete pathologic assessment but carries risks of permanent incontinence (5-15%) and erectile dysfunction (30-60%). Radiation therapy, including proton therapy, avoids surgical risks but has lower rates of incontinence and erectile dysfunction that develop gradually over years. Patient age, baseline urinary and sexual function, and personal preferences guide this decision.
Proton Therapy vs. Active Surveillance: For low-risk prostate cancer, active surveillance with delayed treatment for disease progression represents a valid alternative that avoids immediate treatment toxicity. Proton therapy may be deferred in these patients, with treatment reserved for those demonstrating disease progression.
The American Society for Radiation Oncology and the American Brachytherapy Society provide clinical guidelines that address treatment selection across risk groups. For patients seeking broader context on therapeutic approaches, finding specialized therapy services near you demonstrates the importance of accessing expert providers, a principle equally applicable to cancer treatment selection.
Emerging Technologies and Future Directions
The field of proton therapy continues to evolve with emerging technologies that promise to further improve treatment outcomes and accessibility. Flash proton therapy, which delivers extremely high dose rates (up to 40 Gy per second), may reduce treatment time while potentially decreasing normal tissue toxicity through radiobiologic mechanisms not yet fully understood. Early preclinical studies suggest flash therapy may spare normal tissues more effectively than conventional dose rates, though clinical translation remains in early phases.
Adaptive proton therapy represents another frontier, incorporating daily imaging and plan re-optimization to account for anatomic changes during the treatment course. This approach could further reduce dose to organs at risk by adjusting treatment plans based on daily prostate position and rectal filling.
Compact proton therapy systems, utilizing alternative acceleration techniques such as laser-plasma acceleration, may eventually reduce facility costs and improve accessibility. These systems could enable proton therapy delivery in community cancer centers rather than large specialized facilities, dramatically expanding patient access.
Research published by the American Association of Physicists in Medicine continues to advance technical understanding of proton therapy physics and clinical implementation strategies. Ongoing clinical trials comparing proton and photon therapy outcomes will provide additional evidence regarding long-term efficacy and toxicity profiles.
FAQ
What is the typical duration of proton beam therapy for prostate cancer?
Standard proton therapy for prostate cancer involves daily treatments delivered five days per week for 7-9 weeks, with each individual treatment lasting 15-30 minutes. Some institutions offer hypofractionated schedules with fewer total treatments (5-6 weeks) and higher daily doses. The specific duration depends on the prescribed dose, fractionation scheme, and institutional protocols.
Can proton therapy be combined with hormone therapy?
Yes, proton therapy is frequently combined with androgen deprivation therapy (ADT) for intermediate and high-risk disease. ADT typically begins 2-3 months before radiation therapy and continues for 6-12 months (intermediate-risk) or 24-36 months (high-risk) after treatment completion. This combination improves biochemical control and overall survival compared to radiation alone.
What are the age limits for proton therapy in prostate cancer?
There are no absolute age limits for proton therapy. Treatment decisions are based on overall health status, life expectancy, and treatment tolerance rather than chronologic age. Elderly patients with good performance status and life expectancy exceeding 10 years are appropriate candidates. Conversely, younger patients derive particular benefit from proton therapy’s reduced secondary cancer risk and long-term toxicity reduction.
How does proton therapy affect erectile function?
Erectile dysfunction following proton therapy occurs in 30-50% of patients, depending on baseline sexual function, age, and androgen deprivation therapy use. Proton therapy reduces erectile dysfunction risk compared to conventional IMRT through reduced dose to penile vasculature and erectile tissue. The risk increases gradually over years following treatment and may be partially reversible with phosphodiesterase inhibitors (sildenafil, tadalafil).
Is proton therapy appropriate for all prostate cancer stages?
Proton therapy is appropriate for localized prostate cancer (stages T1-T4, N0, M0) but is not indicated as monotherapy for metastatic disease. For high-risk localized disease with pelvic lymph node involvement, proton therapy can effectively treat the prostate, seminal vesicles, and pelvic lymph nodes in an integrated treatment volume.
What happens if I develop recurrent prostate cancer after proton therapy?
Salvage treatment options for recurrent prostate cancer after radiation therapy include hormone therapy, chemotherapy, and occasionally salvage brachytherapy or cryotherapy. Repeat external beam radiation is generally not recommended due to cumulative normal tissue toxicity. Treatment selection depends on the site of recurrence (local versus distant) and overall health status.
How does proton therapy impact bowel function?
Proton therapy reduces bowel toxicity compared to conventional IMRT through reduced dose to the rectum and small bowel. Acute bowel effects (loose stools, urgency) occur in 20-40% of patients during treatment but typically resolve within weeks. Late chronic bowel effects (rectal bleeding, urgency) occur in 5-10% of proton therapy patients, substantially lower than the 15-25% rate with IMRT.
Proton beam therapy for prostate cancer represents a significant advancement in cancer treatment, offering superior dose distribution and reduced normal tissue toxicity compared to conventional radiation therapy. While cost and facility availability present barriers to widespread access, ongoing technological improvements and expanding evidence of clinical benefit suggest that proton therapy will play an increasingly important role in prostate cancer treatment. Patients should discuss proton therapy options with their radiation oncologist to determine whether this advanced modality is appropriate for their individual clinical situation.


